ISSCR 2019 Poster
Authors
Saba Ghassemi, David Heo, Francisco J. Martinez-Becerra, John Leferovich , Sarah Richman, Alyssa M. Master, and Roddy S. O’Connor
Poster Abstract
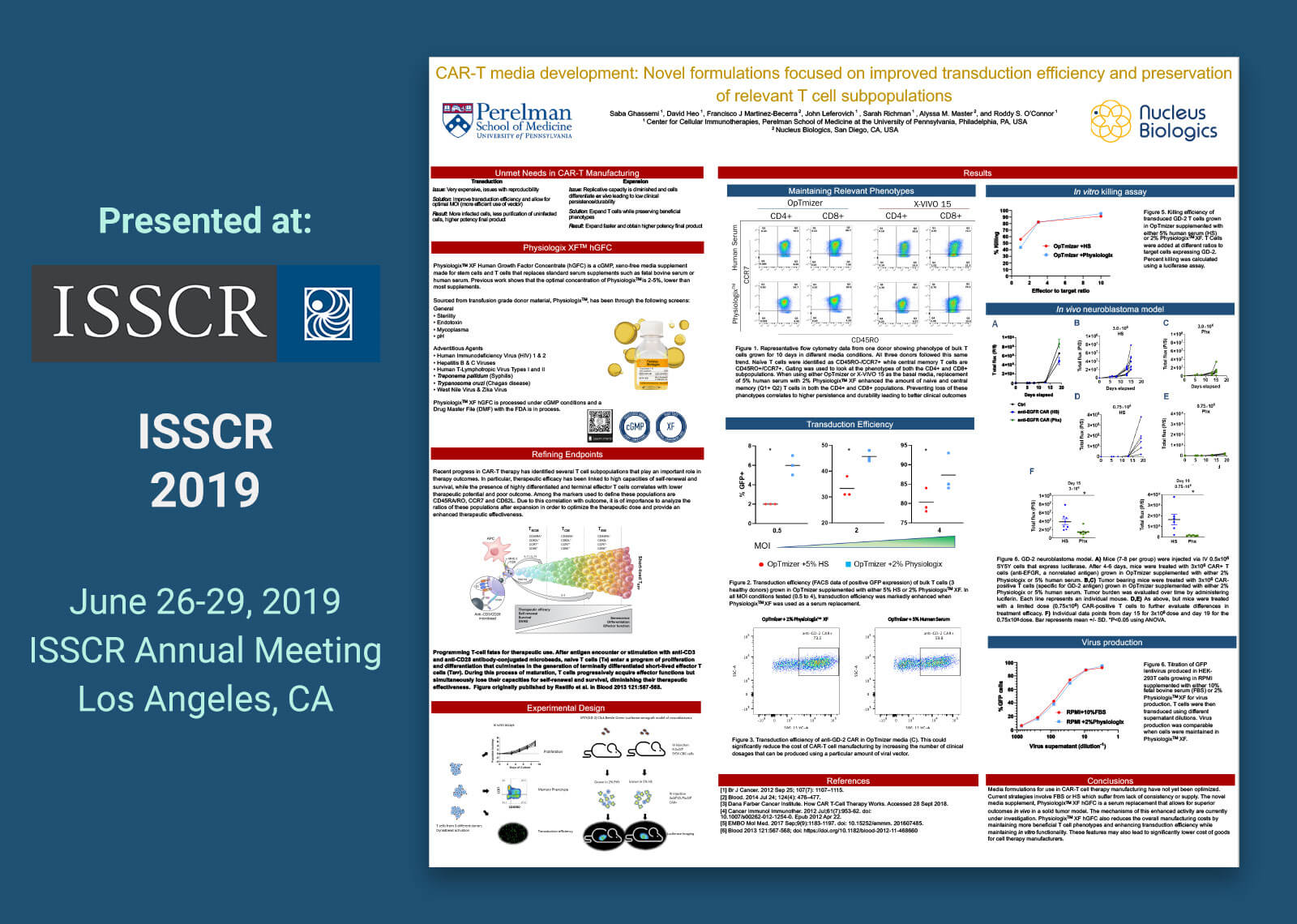
Adoptive immunotherapy involves patient T cell expansion in optimal conditions for the purpose of re-infusing their progeny as therapy. This calls for the generation and characterization of novel media supplements and formulations to boost the progress of the cell therapy market by providing physiologically relevant protein sources in concentrations suitable for biological activity. PhysiologixTM XF Human Growth Factor Concentrate (hGFC) is a cGMP, xeno-free serum replacement that replaces supplements such as fetal bovine serum (FBS) or human serum (HS). In this study, T cell media such as OpTmizer and X-VIVO 15 were supplemented with 5% human serum or 2% PhysiologixTM XF. In addition, RPMI 1640 with 10% FBS was compared due to its prevalent use in non-clinical settings. T cells from three healthy donors were activated with CD3/CD28 Dynabeads and then cultured for 14 days in varying media formulations. Proliferative capacity was assessed with flow cytometry. PhysiologixTM XF showed increased potency relative to human serum when used along X-VIVO 15 or OpTmizer media. Differentiation status was assessed using CCR7 and CD45RO to distinguish T cell subpopulations. We detected an increase in naïve and central memory populations when cells were cultured in PhysiologixTM XF compared to human serum. These phenotypes are highly relevant for the outcome of patients undergoing CAR-T cell therapy. To further define the advantage of our supplement in generating therapy ready products, we compared the transduction efficiency of a lentiviral-GFP in OpTmizer, X-VIVO 15 or RPMI supplemented with 2% PhysiologixTM XF or 5% HS at different multiplicities of infection (MOIs). A higher transduction efficiency was consistently observed when using PhysiologixTM XF at a high MOI of 4 down to a MOI of <1. These results were also observed for anti-GD-2 and anti-EGFR virus. This increased transduction efficiency and preservation of T cell subpopulations can translate into a higher number of transfusable CAR+ cells with relevant phenotypes which allow for reduced overall costs and better clinical outcomes. The ability of anti-GD-2 CAR-T cells expanded in medium supplemented with PhysiologixTM XF to directly kill target cells and control tumor burden in an aggressive leukemia xenograft model is discussed.

 © 2023 All Rights Reserved.
© 2023 All Rights Reserved.